Children’s Health Defense (CHD) today filed a lawsuit in U.S. District Court for the District of Columbia against the Centers for Disease Control and Prevention (CDC) to obtain documents related to the agency’s safety monitoring of the COVID-19 vaccines through the Vaccine Adverse Event Reporting System (VAERS) database.
The lawsuit alleges the CDC violated provisions of the Freedom of Information Act (FOIA) by failing to respond to CHD’s FOIA requests to provide key analysis of reports in the vaccine safety database and intra- and inter-agency communications about those reports.
CHD submitted the FOIA requests in the summer of 2022.
The agency said the requested information would be forthcoming by December of last year, but the documents were never delivered.
CHD is asking the court to compel the release of the requested documents in compliance with the FOIA.
Mary Holland, CHD president and general counsel, explained the significance of the lawsuit to The Defender:
“The CDC continues to ask the American public to believe that COVID shots are safe, without providing the evidence. Our lawsuit demands that the CDC give us hard data to back up its claim. No one should take vaccine safety on faith.”
CDC analysis of VAERS data
The CDC and the U.S. Food and Drug Administration (FDA) jointly administer VAERS, a database that allows healthcare professionals and others to file reports about vaccine-related injuries.
While VAERS reports of adverse events don’t prove causality, the CDC considers VAERS to be a key “early warning system” for detecting unusual or unexpected patterns of adverse event reporting that can signal safety problems with a vaccine.
According to the co-sponsored VAERS “Standard Operating Procedures” (SOP) for COVID-19, dated Jan. 29, 2021, the CDC and the FDA would coordinate monitoring for “potential new safety concerns for COVID-19 vaccines” by performing routine VAERS surveillance.
Each agency would use a different standard approach to data mining to screen for potential safety signals.
The CDC would run proportional reporting ratio (PRR) data mining on a weekly basis, or as needed. PRR would compare the reports of specific adverse events suffered after receiving a Moderna or Pfizer mRNA vaccine to reports made after receiving any other vaccine to see if there is an indication that the COVID-19 mRNA vaccines cause more adverse events than vaccines generally considered by the CDC to be safe.
The SOP stipulated that the FDA would conduct a bi-weekly thorough manual review of serious adverse events and through empirical Bayesian (EB) data mining, which uses a different statistical method to compare adverse events related to the COVID-19 vaccine with those related to non-COVID-19 vaccines in order to identify safety signals.
According to the CDC, if these forms of data mining raise a safety signal, then the agencies would do further analysis to confirm whether the adverse event was caused by the vaccine and that data would be shared with the public.
Both agencies failed to make data public.
Last month CHD filed a federal lawsuit against the FDA to obtain documents related to its empirical Bayesian data mining, The Defender Reported.
In today’s case, CHD made two requests.
The first request sought the records of all PRR conducted by the CDC related to COVID-19 from Oct. 1, 2021, to the present, along with all communications within the CDC and with the FDA about the PRR results and follow-up investigations required by the SOP done in connection with those results.
The second request sought the daily email alerts from VAERS contractors hired by the CDC listing identification numbers for all adverse events of special interest, which are events requiring further investigation.
CDC’s conflicting statements about its VAERS monitoring
Since last year, the CDC has issued a series of conflicting statements to The Epoch Times and CHD regarding their ongoing monitoring of the VAERS database.
In June 2022, the CDC told CHD that “no PRRs were conducted by the CDC” and that data mining was outside of the agency’s purview.
This was despite the fact that the SOP document dated Jan. 29, 2021, stipulated the CDC and FDA would begin ongoing data mining analysis then.
Next, in July 2021, the CDC told The Epoch Times that it had in fact been performing PRRs since January 2021 and was continuing to do so.
Two months later, the CDC changed its story again. This time it told The Epoch Times that it performed PRR analysis for a four-month period, from March 25, 2022, to July 31, 2022.
March 25, 2022, was three days after CHD emailed the CDC reminding them of their pending FOIA requesting the PRR analysis.
At that time, the CDC also said that its PRR results were “generally consistent” with the empirical Bayesian mining the SOP indicated would be done by the FDA and that it had not revealed any unexpected safety signals.
“Given it is a more robust data mining technique, CDC will continue relying upon EB data mining at this time,” the CDC spokesperson told The Epoch Times.
In January 2023, The Epoch Times reported that the CDC provided it with some of the PRR analysis it conducted between March and July 2022. The CDC shared an overall PRR analysis of all events reported from Dec. 14, 2020, to July 29, 2022.
But, it only shared its weekly analysis from three weeks — the weeks of July 15, 22, and 29, 2022. It is unclear whether the CDC did analysis during other weeks, or whether it never did the ongoing analysis mandated by the SOP.
That analysis revealed hundreds of safety signals, including signals for serious conditions such as blood clotting in the lungs, intermenstrual bleeding, lack of oxygen to the heart and even death.
The CDC admitted it gave false information about COVID-19 vaccine surveillance, including inaccurately saying it had conducted a certain type of analysis more than a year before it actually did.
Yet, given its conflicting statements, what surveillance analysis the CDC actually did, and when it did it, remains unclear. Access to this information would reveal when the CDC knew about safety signals related to the COVID-19 vaccine.
CHD’s FOIA request seeks all of these documents in order to clarify this timeline.
CDC anticipated spike in VAERS reports with COVID vaccine
Despite the fact that health officials and scientific journals have downplayed or dismissed the importance of VAERS during the pandemic, the CDC states in the SOP that it is a key “front-line system to monitor the safety of vaccines licensed for use in the United States.”
Information revealed through FOIA requests demonstrates that the CDC was anticipating a spike in VAERS reporting when the new vaccines were released and spent millions of dollars hiring contractors to process the data. Communication with these contractors is part of CHD’s FOIA lawsuit.
A FOIA request revealed, in late August 2020, that the CDC contracted with General Dynamics to handle VAERS reports for COVID-19 vaccines. The contract anticipated up to 1,000 reports per day, with up to 40% of them serious in nature, Josh Guetzkow reported. The value of the year-long contract was $9.45 million.
“This means that months before the EUA [emergency use authorization] of any COVID vaccines, the CDC anticipated up to a 600% increase over the average annual number of VAERS reports in recent years with 8 times the rate of serious reports,” Guetzkow said in his report.
In March 2021 the contract was amended to process backlogged reports filed through Feb. 28 for an additional $21.5 million. The CDC also contracted Eagle Health Analytics to assist with the processing for an additional $6 million.
Although other financial information about the dollar amounts associated with the contracts was redacted, Guetzkow estimated that the CDC paid contractors at least $40 million over two years to process VAERS data.
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed. Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
As of Feb. 3, 2023, a total of 1,517,779 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and Feb. 3, 2023, to VAERS.
The data included a total of 34,270 reports of deaths and 279,669 serious injuries, including deaths, during the same time period.
There were a total of 21,954 reports of adverse events following the new COVID-19 bivalent booster as of Oct. 21, 2022. The data included a total of 173 deaths and 1,458 serious injuries. As of Feb. 8, 52.5 million people have received the updated bivalent booster dose.
Of the 31,696 reported deaths, 21,479 are attributed to Pfizer’s COVID-19 vaccine, 9,638 to Moderna’s, 2,944 to Johnson & Johnson’s (Janssen) and 18 to Novavax, according to multiple sources including Albert Benavidas, a data analyst who runs VaersAware.com.
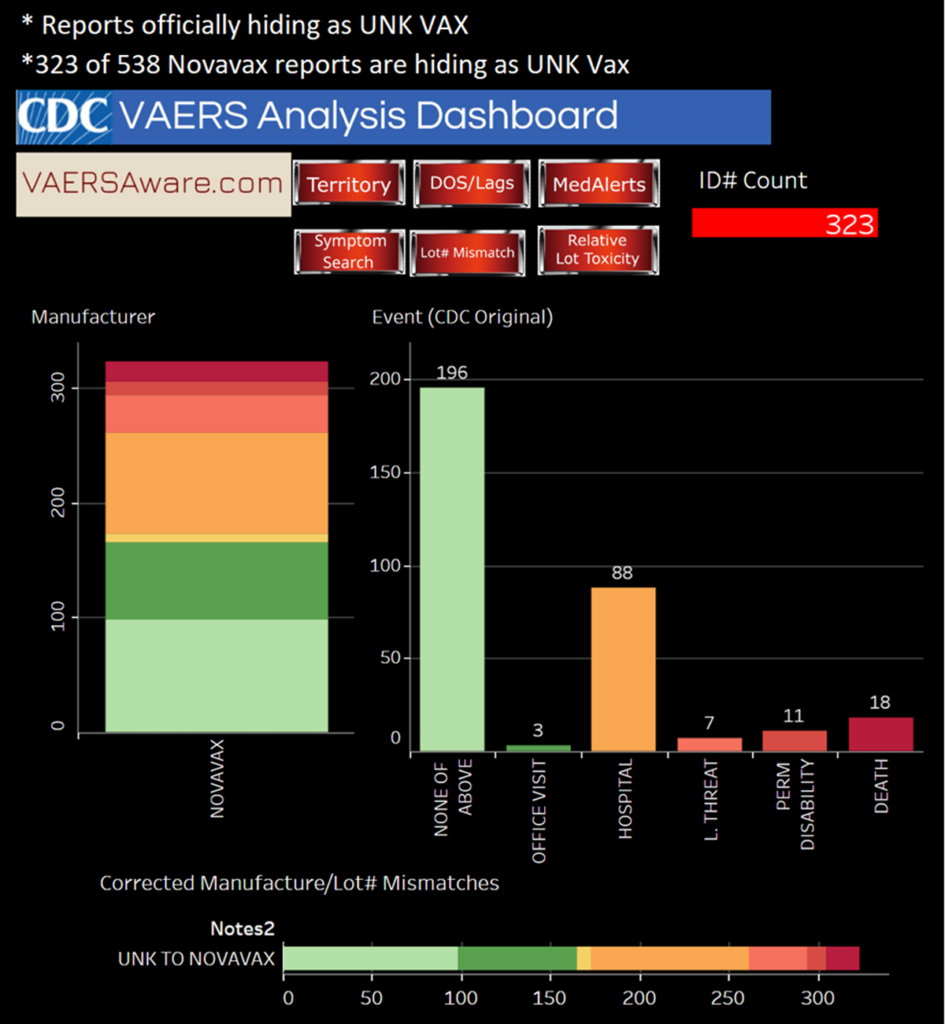
Updated: This article was updated to include information showing that 18 deaths have been reported following administration of the Novavax vaccine.








