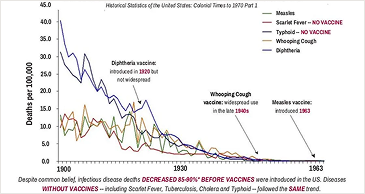
Why Informed Consent Matters
The topics of vaccine safety and the inherent need for true informed consent have never gotten as much attention as they have since the COVID crisis – and, with ever-increasing numbers of vaccines on the childhood schedule – these issues have never been more important. Only a thorough understanding of the risks and benefits of each vaccine required for school attendance can provide families with genuine informed consent. Unfortunately, this is not typically what parents receive at routine pediatrician visits. It’s incumbent upon parents and caregivers to gain this knowledge by researching vaccines and asking critical questions of medical practitioners before consenting to vaccines for their children.

Informed consent is a foundational human and civil right, affirming that any medical intervention, vaccination included, must be accompanied by full disclosure of its potential benefits, risks, and uncertainties, allowing individuals to make voluntary, knowledgeable decisions.
Vaccines, like any pharmaceutical product, carry risks of injury or death – risks which cannot always be predicted beforehand; thus, individuals must have the information and freedom to refuse without coercion or penalty.
Rooted in ethical principles established after the Nuremberg Trials and enshrined in modern medical law, informed consent encompasses the right to understand all aspects of treatment, including known and unknown effects, available alternatives, and the voluntary nature of acceptance. This ethical standard emphasizes respect for bodily autonomy and self‑determination, making it essential that healthcare decisions be made transparently and without undue influence.
School Exemption Laws by State
U.S. states require children to receive certain vaccines before attending school, typically adopting the CDC’s childhood vaccine schedule (which has risen from 5 doses by age 18 in 1962 to as many as 85 doses today). However, all states allow medical exemptions, and most states also provide exemptions for families who decline immunization for non-medical reasons.
These exemptions typically fall into two categories:
- Religious, for families whose faith opposes vaccination
- Philosophical or personal belief exemptions, for those who object based on moral or other personal grounds
The availability and requirements for these exemptions vary by state, with some enforcing stricter rules, such as educational counseling or notarized documentation, while others offer more flexibility.
Currently, five states – New York, Maine, Connecticut, West Virginia, and California – do not allow for either religious or philosophical exemptions. While these states do provide medical exemptions, proving the need for these can be daunting and doctors who provide them often face retribution from government officials and state medical boards.
https://www.ncsl.org/health/state-non-medical-exemptions-from-school-immunization-requirements
https://www.vaccinesafety.edu/vaccine-exemptions/
https://www.nvic.org/resources/frequently-asked-questions/vaccine-exemptions
For assistance in applying for a religious exemption, the Pacific Justice Institute provides these straightforward instructions about the process. If your application has been denied, the Institute also provides these instructions about what to do next, along with links to the relevant employment discrimination agencies in every state.
Understanding Risk vs. Benefit
When parents bring their children in for “well child” visits, pediatricians typically provide Vaccine Information Statements (VIS) that include basic information for each vaccine. These statements, however, are very general and focus on inconsequential reactions such as redness and soreness at the injection site. The lack of detail and the minimization of serious adverse events does not provide caregivers with enough information to properly understand the risk/benefit ratio.
Parents and caregivers need to look further than the VIS and read the package insert for each vaccine their children are being asked to take. Package inserts include detailed information on adverse reactions during clinical trials and in the postmarketing phase as well as the duration of follow-up the children who participated in the trials received. The product insert for GlaxoSmithKline’s Engerix Hepatitis B vaccine, for example, reveals that children in the clinical trials were monitored for reactions for a mere four days after receiving the vaccine.
For more in-depth information about vaccine product inserts, see our two-part eBook, Read the Fine Print: Vaccine Package Inserts Reveal Hundreds of Medical Conditions Linked to Vaccines and Read the Fine Print, Part Two— Nearly 400 Adverse Reactions Listed in Vaccine Package Inserts.