Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The U.S. Food and Drug Administration’s (FDA) vaccine advisory panel met Wednesday to discuss the topic of a second booster dose of COVID-19 vaccines for the American public.
After nine-and-a-half hours, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) failed to reach a consensus, even though last week — despite a paucity of data — the agency authorized a second booster dose for people over age 50 and the immunocompromised.
The committee did not discuss Emergency Use Authorization or approval of COVID vaccines for children or infants.
In a session riddled with technical issues and punctuated by a sobering 60-minute long public hearing, committee members sought to answer these basic questions:
- Is a second mRNA vaccine booster effective?
- How should effectiveness be defined?
- Can we predict what future variants will look like and when they will emerge?
- At what point will a new vaccine formulation be needed?
After more than four hours of scheduled presentations from researchers in the U.S. and Israel followed by a prolonged discussion, the committee answered the above questions as follows:
- Maybe, but not for very long
- By a yet-to-be-determined reduction rate in hospitalizations
- Not really
- It cannot be known until more data is available
Dr. Meryl Nass, an internist and member of the Children’s Health Defense Scientific Advisory Committee, live-blogged her impressions of the discussion here.
Effectiveness of second booster: where’s the data?
Two hours into Wednesday’s meeting, Dr. Sharon Alroy-Preiss, director of Public Health Services, Israel, and Ron Milo, Ph.D., presented the only outcome data available on the effectiveness of a fourth dose of the Pfizer vaccine.
This was the data that prompted the FDA to act more than a week before this meeting was convened.
The government of Israel offered the second booster to Israelis over age 60 (not 50) at least four months after their third dose.
Approximately one-half of the over-60 population in Israel was doubly boosted.
With 1.2 million Israelis in this age group, this New England Journal of Medicine (NEJM) study compared two groups of about 600,000 individuals. This population was observed for only eight weeks.

The above graph, published April 5 in the NEJM, was a key part of Milo’s presentation. Note that the y-axis is Adjusted Rate Ratio (ARR), not vaccine effectiveness. Vaccine effectiveness can be defined as: 1 – (1/ARR).
The effectiveness of the second booster in preventing infection (blue) peaked at week three and waned to 9.1% by week eight. In contrast, with regard to severe infections (red) the effectiveness peaked at week six (71%).
Milo then presented non-peer-reviewed data that showed effectiveness against severe disease declined substantially (56%) at week seven. This point was not stressed and committee members did not question him further about it.
Notably, “severe illness” was not defined as hospitalization but as one of three clinical findings: a respiratory rate >30, oxygen saturation less than 94% or a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300.
These clinical findings are indicative of significant respiratory compromise but do not necessarily constitute a need for hospitalization.
The key points here are:
- Vaccine effectiveness began to wane very early after the second booster (four weeks with regard to infection and seven weeks with regard to severe illness).
- Severe illness, as defined in the study, was different from the committee’s consensus position on what constituted a measurable outcome to determine efficacy (hospitalization).
Rapidly emerging variants — do we need new vaccines?
Prior to the reports from the Israeli researchers, Centers for Disease Control and Prevention (CDC) employee Heather Scobie, Ph.D., MPH, presented the CDC’s information on emergent SARS-CoV-2 strains in the U.S.
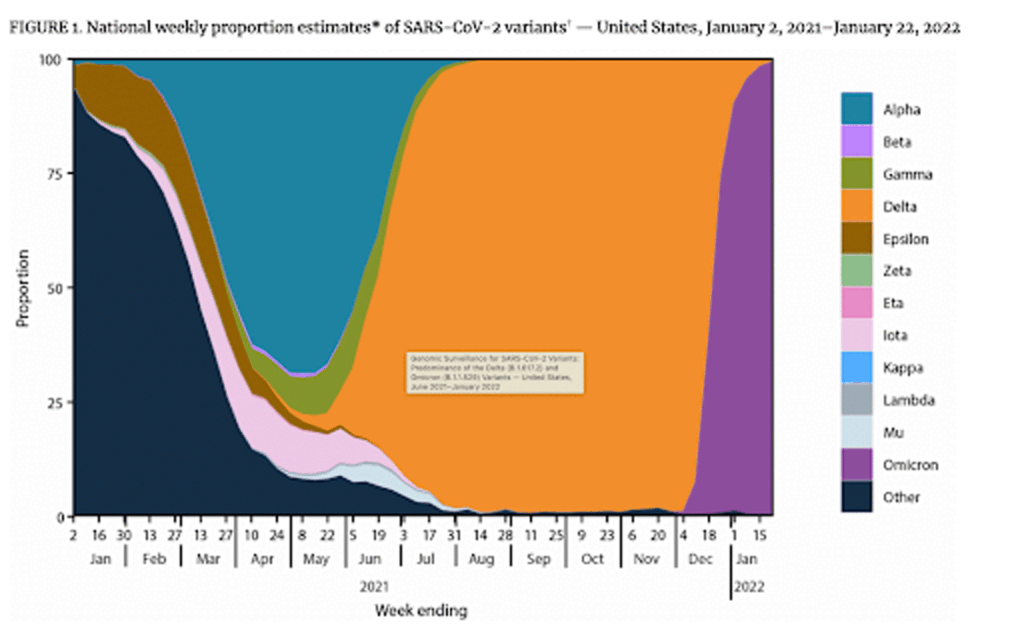
The above plot demonstrates the rapid emergence over time of variant strains in the U.S.
As of the beginning of 2022, the Omicron variant is responsible for the overwhelming majority of cases, hospitalizations and deaths in this country.
This makes the strategy of continued boosting with a vaccine formulation designed to protect against the original Wuhan strain questionable.
One advantage of using mRNA-based vaccines is that new formulations can be rapidly developed. However, even if a new variant is identified, it still takes months to manufacture, test and deploy.
Robert Johnson, deputy assistant secretary at the Biomedical Advanced Research and Development Authority , who presented during the meeting said:
“If you’re not on your way to that clinical trial by the beginning of May, it’s going to be very difficult to meet demand [by September].”
The FDA is in a difficult position. New variants will emerge. The current vaccines show waning efficacy within a few months. When will it be appropriate to authorize a new primary series of reformulated vaccines?
Nass summed it up this way:
“The FDA has no idea what it wants nor what it will do regarding planning for new versions of boosters. None of its speakers are willing to venture a guess as to what will be needed. Of course, all are scared that they will recommend some variant that will have disappeared before the vaccine rollout.”
Dr. Cody Meissner, a VRBPAC committee member and chief of pediatric infectious diseases at Tufts University, asked what was perhaps the most obvious question regarding the emergence of variants: “Why are we seeing so many?”
His question was not answered. There was no discussion of the theory that introducing highly specific, leaky (those that do not prevent infection or transmission) vaccines in the midst of a global pandemic will necessarily result in resistant strains.
Geert Vanden Bossche, DVM, warned authorities of this inevitability last year.
Public, vaccine-injured weigh in
The committee heard 21 separate three-minute presentations from members of the public.
These included:
- Jessica Rose, Ph.D., who expressed her dismay that at least one of the committee’s temporary voting members had a financial conflict of interest.
- Statistician Mathew Crawford, who unequivocally stated there is “no transparent data that demonstrate any efficacy of the experimental COVID-19 vaccines” by offering numerous examples of bias and improper analyses in trial data and subsequent studies. He also reminded members of clear safety signals coming from the Defense Medical Epidemiology Database.
- David Wiseman, Ph.D., presented a flurry of data from the CDC, Israel and elsewhere demonstrating rapidly declining vaccine efficacy and increased all-cause mortality in the vaccinated. He raised additional questions about the evidence of reverse-transcription of vaccine mRNA and the persistence of spike protein in the vaccinated.
- Peter Doshi, Ph.D., associate professor of Pharmaceutical Health Services Research at the University of Maryland School of Pharmacy and editor at The BMJ, reminded the committee of Brook Jackson, a clinical trial coordinator and whistleblower who offered proof of data manipulation at one of Pfizer’s trial sites.
- A young woman who nearly died from COVID expressed her support for the vaccination program.
- Josh Guetzkow, Ph.D., reported on the enormous underreporting factor of adverse events in Israel.
- Rebecca Rotem, the mother of a fully vaccinated 12-year-old who subsequently caught COVID asked if it is safe for him to get boosted. With no safety data regarding children in his situation, why are summer camps insisting that he get boosted in order to attend?
Additionally, more than a dozen individuals told personal stories of how COVID vaccines devastated their lives and how the medical system and the government ignored their pleas for help.
Brianne Dressen, a young woman who was severely injured in the Astra-Zeneca COVID vaccine trial and ignored by the investigators and vaccine manufacturer.
Committee members refrained from acknowledging the outstanding research offered by the independent scientists or the plight of those who were injured by the jabs.
More takeaways
An extended discussion period followed the last scheduled presentation. Temporary committee member and chair of the meeting, Dr. Arnold Monto, sought to establish a consensus position on how to proceed.
In summary:
- Many members and presenters agreed that antibody levels are not a valid measure of immunity, i.e. a correlate of protection. Thus, the only way to determine vaccine effectiveness is through a clinical trial.
- There is not really time to do a clinical trial to pick a strain or strains by June to make a vaccine that would target said strains available for the fall.
- Dr. Peter Marks, director of the FDA’s vaccine division, Center for Biologics Evaluation and Research, admitted the fourth booster dose approved last week was a “stopgap measure” — in other words, a temporary measure to be implemented until a proper solution may be found in the future.
- Despite the equivocal findings from Israel, claims were repeatedly made that the present vaccine still protects against severe outcomes and death, but FDA’s lead scientist for this presentation, Doran Fink, admitted efficacy is also waning for severe outcomes.
No data was presented at all regarding strain choice/prediction of what to use as the antigen(s) for a newer vaccine.
Commenting on that lack of data, Nass said:
“It was as if everyone just got the idea to begin thinking about this yesterday. It appeared that neither CDC, FDA nor the VRBPAC advisors wanted to take any initiative or responsibility in figuring out what kind of a vaccine comes next.
“Was everyone dancing around the strain/variant choice because in fact no one really wants a newer vaccine, or because no one wanted to be responsible for picking a loser? I could not tell if this was a slow-roll or an agency and advisors who are highly risk-averse.”
The committee will meet again early this summer after more data becomes available.







