Miss a day, miss a lot. Subscribe to The Defender's Top News of the Day. It's free.
The U.S. Food and Drug Administration’s (FDA) vaccine advisory committee met this week to discuss requests to amend the Emergency Use Authorization (EUA) of the Moderna and Pfizer-BioNTech COVID-19 mRNA vaccines.
Moderna asked for its EUA to include the administration of a primary series of the vaccine to infants, children and adolescents ages 6 months through 17 years.
Pfizer-BioNTech asked that its EUA include the administration of a primary series to infants and children 6 months old through age 4.
Vaccinations and/or boosters in these age groups are unnecessary.
However, based on the Vaccines and Related Biological Products Advisory Committee’s (VRBPAC) extended history of ignoring fundamental aspects of immunology, drug safety and epidemiology — occurring as recently as last week with its approval of the Novavax COVID-19 vaccine — it was always a foregone conclusion that the committee would approve these proposals despite the significant risk to children and noteworthy lack of effectiveness.
In fact, the White House was so confident the FDA committee would authorize the vaccines, officials weeks ago announced plans to begin injections in kids as early as June 21.
Interestingly, while touting how “safe and effective” the vaccines are for children, FDA employees are all calling into the VRBPAC advisory committee meeting remotely, putatively using the COVID-19 pandemic as their reason — isn’t that more than just a little ironic?
Epidemiological evidence doesn’t support COVID shots for infants, children and adolescents
Epidemiological evidence shows infants, children and adolescents never needed COVID-19 vaccines and certainly do not need them now.
According to the Joint Committee on Vaccination and Immunisation (JCVI), 4 million doses must be administered to children 5 to 11 years old to prevent a single ICU admission.
Assuming two doses per child, that means 2 million children must risk potentially serious side effects to prevent a single child from requiring intensive care due to COVID-19.
“Vaccination of children aged 5 to 11 years who are not in a clinical risk group would prevent a relatively small number of hospitalizations or intensive care admissions.
“For a variant like Omicron, it would take around four million vaccine doses to two million children to prevent one admission to ICU. For less severe illnesses, 58,000 child vaccinations would prevent one child hospitalization.
“Children admitted recently to hospital with COVID had an average length of stay of 1-2 days. The Omicron wave saw no more children in hospital than before Omicron hit the UK.”
When deciding whether to approve the EUA requests there are two central questions that VRBPAC must not ignore.
The first is whether vaccination of children is even needed at all.
It is no longer summer 2020. We are no longer deeply embedded in the throes of the pandemic.
It is well established that children, even without vaccination, have low risk of serious COVID-19 complications.
Primary approval for young children is not the same as the emergency approval of COVID-19 vaccines for adults in 2020.
The risk COVID-19 presents to children is minuscule. Medical literature plus multiple articles in the lay press have detailed for some time, and in no uncertain terms, that it’s hard to justify vaccinating the younger age group at all because severe disease and hospitalizations in unvaccinated children are so rare.
Knowing that, one must wonder: Why on earth, to this very day, is the FDA’s homepage picturing and pushing for young adolescents and little kids to get the vaccine?
The vaccines we are talking about were developed for the original strain of COVID-19, which is responsible for fewer than 1% of new cases.
COVID-19 mutations are much less severe, and there is widespread availability of preventative, early-exposure and early-treatment therapeutics with known safety records, not to mention protective measures such as mask-wearing and social distancing.
The second question is whether children who have naturally acquired immunity through previous infections should be vaccinated.
A recent study sponsored by Moderna and Dr. Anthony Fauci’s National Institute of Allergy and Infectious Diseases found natural immunity is superior to immunity conveyed by any COVID-19 vaccine.
VRBPAC members also seemingly deliberately ignored natural immunity in recommending primary series of vaccines in children, despite a recent Johns Hopkins study which found 99% of all COVID-19 infections resulted in natural immunity antibody expression that persisted for up to 20 months following infection.
Since we do not have a full accounting of safety, especially long-term risks in adults, it is inappropriate to propose mass vaccination in children, especially those who have already recovered from COVID-19.
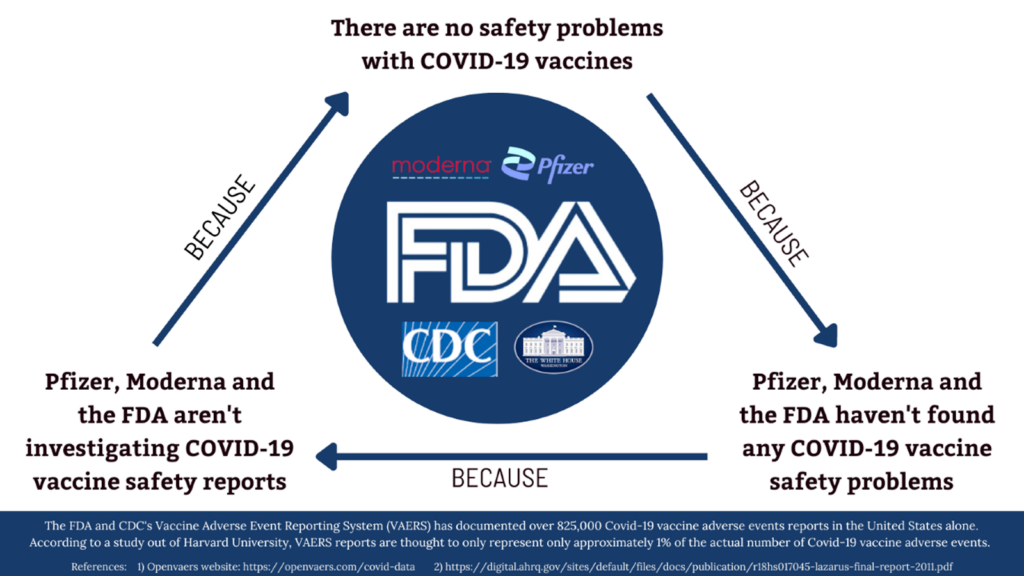
More than 12 billion doses of COVID-19 vaccines have been administered worldwide. In the U.S. alone, more than 825,000 COVID-19 vaccine-related adverse events have been reported.
According to a Harvard University study, that figure is estimated to represent only approximately 1% of the actual number of COVID-19 vaccine adverse events.
Of particular note, many of the more serious cardiovascular adverse events from COVID-19 vaccinations and boosters disproportionately affect a younger population.
Another independent analysis shows children under 18 are 51 times more likely to die from the vaccine than they are to die from COVID-19 infection if not vaccinated — more reasons to be cautious before giving emergency vaccine approval for children.
COVID-19 vaccines are ineffective against new variants
VRBPAC must not fall for the fallacy of “mild disease if vaccinated” or “it would have been severe disease without vaccination/boosters.”
Data show it’s not just “boostered” people who have mild COVID-19 symptoms. Essentially everyone — regardless of COVID-19 vaccination status — will have less severe disease. That is the typical pattern of viral mutations.
In other words, in addition to a serious safety risk, there is no clinical, statistical or epidemiological benefit of vaccination in this particular group.
Despite mild symptoms and the high drug safety risk, the director of the FDA’s Center for Biologics Evaluation and Research said he would not hold up approval for efficacy, even if it was lower than the 50% efficacy threshold of preventing severe disease, as required in official FDA guidelines.
It breaks all FDA norms and practices for the agency to leap into an EUA so blindly and ignore decades-old standards of making careful safety- and efficacy-based decisions, especially when we are talking about our children.
Leaving the bioethics argument and question of using our children as test subjects aside, whatever happened to using hard clinical and scientific evidence as the basis for making decisions?
What about the FDA-employed physicians plus the physicians who serve on VRBPAC and their centuries-old sacrosanct vow to “First, do no harm?”
The abandonment of the FDA’s decades-old efficacy and safety standards is part of the White House’s unrelenting “data be damned” push for COVID-19 vaccines to our kids no matter what.
It is time for the FDA advisory committee members to stop blindly listening to federal agencies, the White House and mainstream news narratives for advice on clinical pharmacology and instead use their credentials, start their research from scratch, and conduct an unbiased review of the safety, efficacy and naturally acquired immunity data themselves.
In summation, there is no need for a primary series of COVID-19 vaccines for children. The young have a decreased benefit from COVID-19 vaccination, but are also at a greater safety risk.
Many children have already had COVID-19 and have the benefits of naturally acquired immunity.
VRBPAC should rely on the fundamentals of clinical science and carefully examine and follow the data — not politics.
America’s children deserve nothing less.





